July 10, 2023 • 4 min read
Clinical Trials Get New GS1 Application Standard
CATEGORIES
SOCIAL SHARE
Utilizing these standards, CodeREADr’s edge computing technology can help improve the safety of research subjects.
Background
GS1 announced a new Application Standard for the identification and barcoding of investigational products used in clinical trials. The standard is based on the collaborative efforts of 60 representatives from 37 different clinical trial organizations worldwide. In close partnership with GS1, this industry-led working group met weekly for nine months to produce the standard.
According to GS1, the standard will streamline processes for all parties involved in clinical trials and allow for efficient data capture methods that leverage barcodes. Our CodeREADr team proposes edge computing technologies can also use this standard to help improve the safety of participating research subjects.
Edge Computing
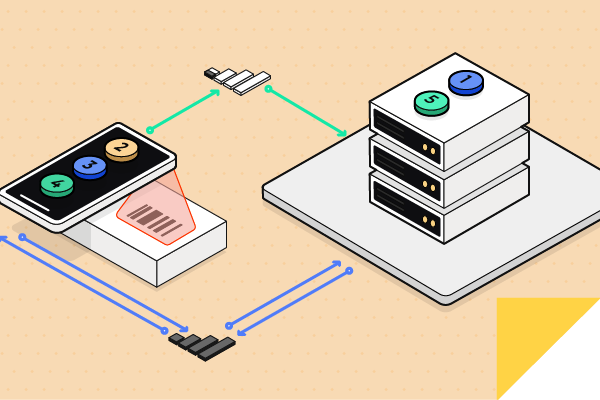
What is Edge Computing and why does it matter?
According to Accenture, “Edge computing is an emerging computing paradigm which refers to a range of networks and devices at or near the user. Edge is about processing data closer to where it’s being generated, enabling processing at greater speeds and volumes, leading to greater action-led results in real-time.”
In the context of the application standard and the goal to increase subject safety, edge computing is important first and foremost for the ability to present actionable data in real-time to the clinician. To do that, scripts hosted on the edge device analyze standardized data captured in real-time. The application standard provides the data structure needed to compute the validation and present the results to the clinician via a secure mobile app.
Safety Benefits with Structured Data
The following benefits are possible with structured data.
- Enrollment Validation: The clinician scans the research subject’s barcode ID. The app validates the ID against an on-device ID database representing the subjects enrolled in the trial (no PII required). This instantly verifies to the clinician that the subject is an authorized subject.
- Duplicate Dose Warning: The clinician is instantly warned if the ID has already been scanned within a specific interval. This verifies that the subject isn’t getting an unintended second dose.
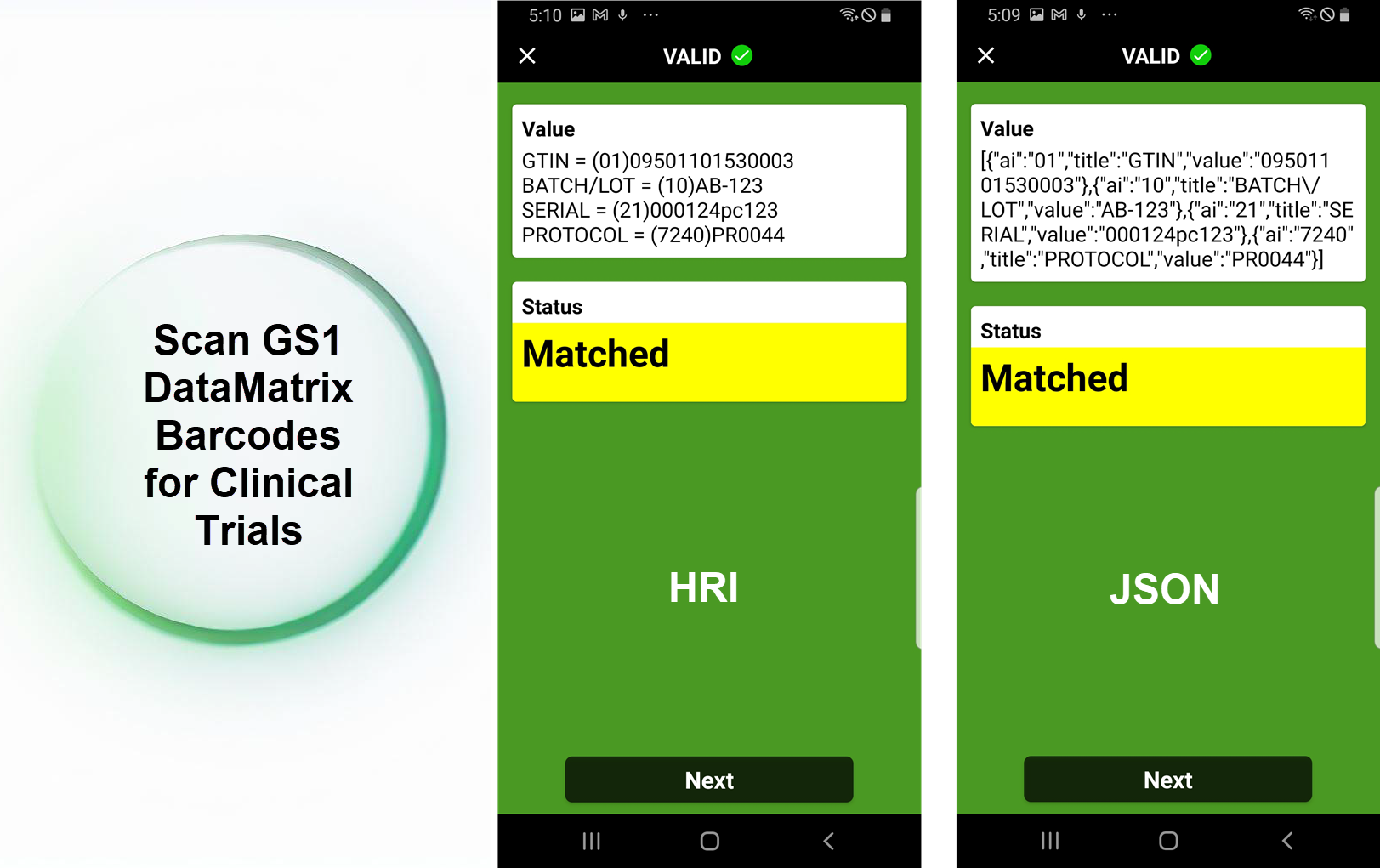
- Drug Verification: In the same scanning session, the clinician also scans the GS1 Data Matrix barcode. The GTIN or Protocol Number is compared to the GTIN or Protocol Number in the on-device database for that specific subject. The clinician is instantly warned if they don’t match. This helps prevent the clinician from giving the wrong drug.
- Dosage Verification: Also in the same scanning session, the clinician can enter or select the dosage they will be administering to the subject. If the entered dosage doesn’t match the prescribed dosage in the database, the clinician will be warned of the error.
The End Goal
Embracing new technology leads us towards a future where clinical trials are more efficient, accurate, and safer, paving the way for faster delivery of treatments and therapies to those who need them most. We thank GS1 and all the participants for their dedication to that end.
In Future Blogs
Additional benefits of this application standard include traceability and inventory management. It starts with the manufacturer who produces the investigational product and extends to the packaging site, distribution center, and clinical sites. This can lead to further improvements in patient safety as well as better control, management, and use of product inventory.
There’s more to follow in future blog posts!