Feb 17, 2026 • 5 min read
Audit Medical Device Safety with GS1 Data Matrix Barcode Scanning

CATEGORIES
SOCIAL SHARE
Use Smartphones, Tablets, and Rugged Mobile Computers to Audit Medical Device Safety with GS1 Data Matrix Barcode Scanning
Medical device safety no longer depends solely on manufacturer labeling or post-incident reporting. In modern hospitals, safety depends on real-time validation at the point of use or through periodic audits. The systems that enable that validation are increasingly within hospital IT control.
Hospitals today must ensure that medical equipment is:
- Correctly identified
- Not expired
- Properly calibrated
- Not subject to recall
- Used only in appropriate contexts
As hospitals standardize on Android and iOS devices, including handheld, portable, or mounted in kiosk mode, IT teams are uniquely positioned to turn those devices into active safety controls, not just data entry tools.
GS1 DataMatrix: The Safety Backbone of Medical Device Identification
Most regulated medical devices are labeled with GS1 DataMatrix barcodes in accordance with:
- GS1 standards
- FDA UDI requirements
A single DataMatrix barcode can encode:
- Device Identifier (DI)
- Lot or batch number
- Serial number
- Expiration date
- Manufacturing date
From a safety perspective, this means critical attributes needed to help prevent device-related harm are available at scan time.
Medical Device Safety at the Edge
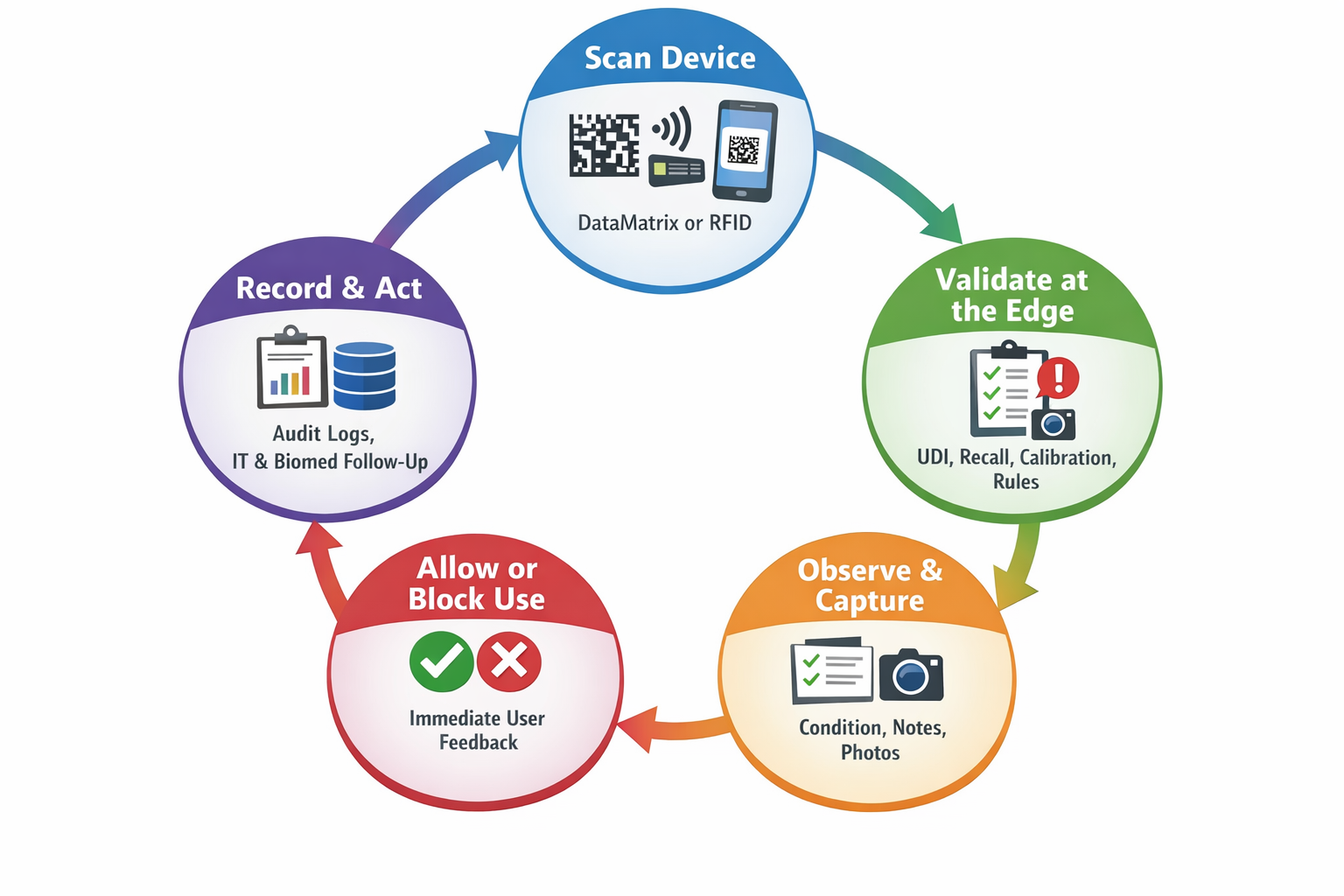
CodeREADr enables both online and on-device (edge) validation on Android and iOS devices, allowing safety decisions to be made immediately, even when connectivity is limited or unavailable.
When a device’s DataMatrix barcode is scanned:
- GS1 Application Identifiers are parsed locally
- Safety rules are evaluated on the device
- The app user receives instant feedback:
✅ Safe to use
⚠️ Warning (e.g., calibration due soon)
❌ Blocked (expired, recalled, incorrect device)
- The app user receives instant feedback:
This helps prevent unsafe equipment from being used before it reaches a patient.
Core Safety Scenarios Hospital IT Must Support
Identifying the Use of Recalled Devices
Recall information often exists in emails, portals, or backend systems—but not always where care happens. With CodeREADr:
- Recall lists can be synced to mobile devices in the background
- Lot and serial numbers are checked locally or online
- Recalled devices are flagged immediately
The expected result is that recalled equipment is intercepted before patient exposure, with a complete audit trail demonstrating compliance.
Enforcing Calibration and Maintenance Compliance
Calibration failures are a preventable safety risk. CodeREADr workflows can enforce rules such as:
- Calibration date must be valid
- The device must not be quarantined
- Inspection status must be approved
If a device fails validation:
- The app blocks usage
- The failed safety check is logged
- IT and biomedical teams gain visibility into near-miss events
Ensuring the Right Device Is Used in the Right Context
When similar devices are stored together or moved between departments, human error becomes a risk factor. CodeREADr can validate:
- Device class
- Department or location assignment
- Workflow or procedure compatibility
This adds a quiet safety layer that supports staff without slowing them down.
Photo Capture for Evidence, Audits, and Follow-Up
Safety does not stop at pass/fail validation. Hospitals also need documented evidence when something appears wrong. After scanning a DataMatrix barcode, CodeREADr can prompt the app user to:
- Report device condition (OK / damaged / needs inspection)
- Enter structured safety observations
- Confirm cleaning or sterilization status
- Add comments or notes
- Capture one or more photos of the device
This transforms a scan into a safety inspection event.
Why This Matters
By combining GS1 DataMatrix and structured data capture on mobile devices, hospitals can:
- Help prevent unsafe device use
- Intercept recalls before harm occurs
- Enforce calibration compliance
- Document device condition at the moment it matters
- Build defensible safety and compliance records
Hospital IT can transform everyday mobile devices into real-time medical device safety terminals.